Why utilizing lime should no longer be an option
The performance of Virotec’s Bauxsol™ Technology and long-term stability has been well documented, peer reviewed and commercially accepted over the past 20 years. Trace-metal removal by Virotec’s reagents are not a simple direct adsorption or hydroxide precipitation, but rather involves the development of new complex and low solubility minerals, isomorphic substitution, co-precipitation, and solid state diffusion. Consequently, Virotec’s Bauxsol™ Technologies and associated Reagents are the safer alternative for traditional Lime (Hydrated or Quick) users due to both short and long-term benefits.
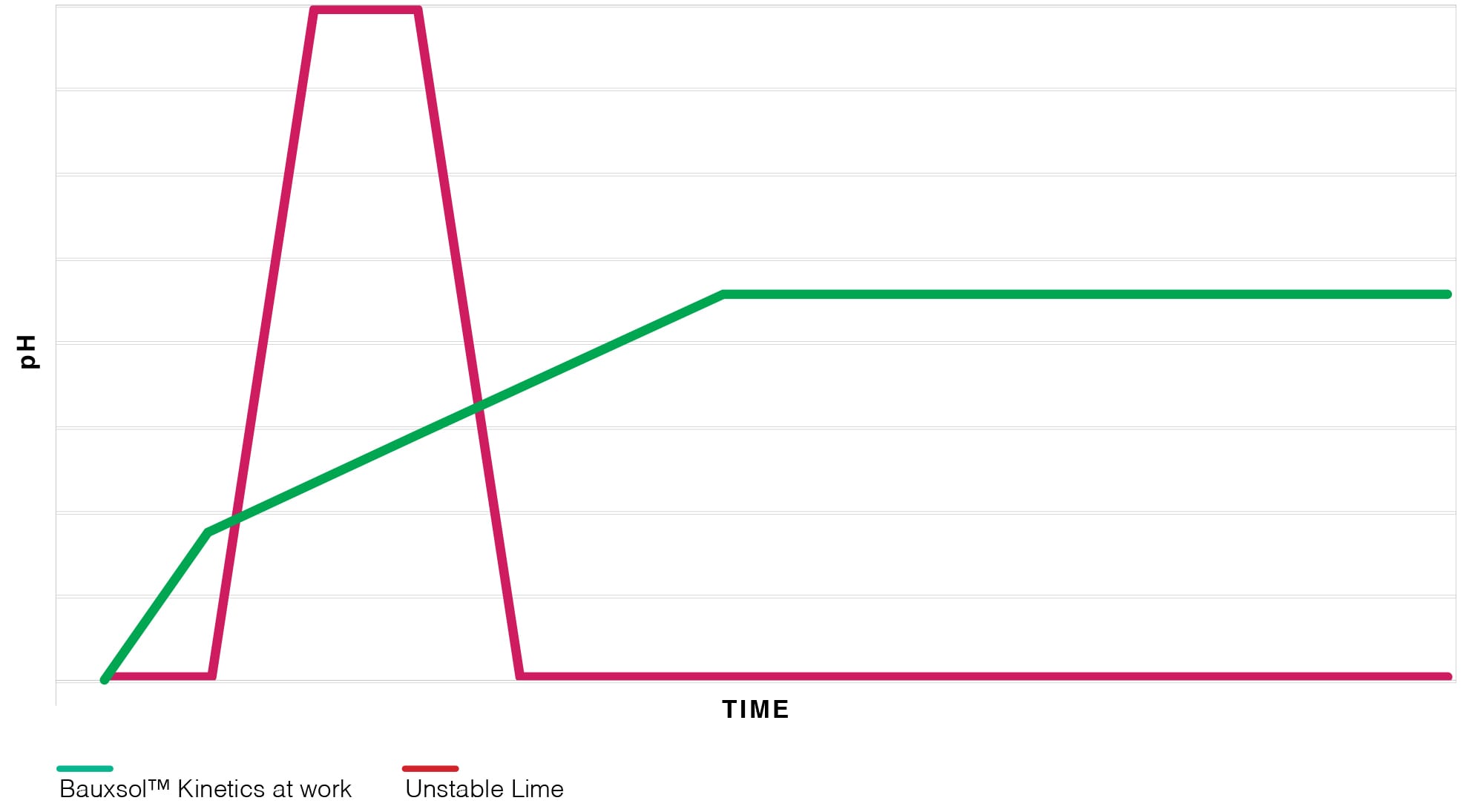
Traditionally, Lime has an image of being “good for acid neutralizer” and perceived to offer better efficiency for polluted soil and wastewater treatment due to its rapid dissolution and ubiquitous availability.
Unfortunately for Lime, the rapid boost of short-term pH adjustment offers little long-termbenefits, low trace-metal removal stability and typically produces a voluminous problematic sludge; lime is also burdened with a significant CO2 out-put and therefore a high Carbon Footprint.
Lime production is carbon intensive as CO2 is released from limestone in the production process (786 kg of CO2 per ton of Quick-lime, 603 kg per ton of Hydrated-lime). Furthermore, the energy generation necessary for converting the limestone, generates significant additional CO2 emissions. Consequently the are 2 carbon foot-prints to consider when using lime.
Virotec’s technology and long-term proven results show that the slow and steady kinetics of Bauxsol™ consistently out-performs Lime’s shot in the dark time after time.
Disadvantages of Using Lime
- Unstable and often provides uncontrollable pH spikes and pH adjustments;
- Highly soluble lime leaches away from site before treating the long-term issue;
- Application rates are critical to performance and even slight over or under treatments can lead to ineffectual results;
- Bicarbonate alkalinity generated with lime catalyses’ pyrite decomposition;
- Gypsum formation causes hydraulic reduction and blockages in passive reactive barrier technologies;
- Surface coating forms using granular lime / deactivation particularly in passive reactive barrier technologies, this requires ultra-fine milling to avoid;
- High volume is required for a temporary treatment solution;
- Additional loading times required due to the extra volume;
- Significant Carbon Footprint;
- Creates a metal laden gelatinates sludge requiring further treatment or disposal;
- High reaction pH’s (>12.0) poses a substantial health and safety risk, often requiring operators to have hazardous materials training;
- Binds many trace-metals, but more-often-than-not fails TCLP Standards;
Benefits of Using Bauxsol™
- Provides a steady and controllable pH adjustment;
- Low solubility therefore can treat the long-term issue;
- Application rates are less critical to performance where slight over or under treatment stil lead to positive treatment results;
- Free alkalinity generated with Bauxsol™ does not catalyses’ further pyrite decomposition;
- Hydraulic reduction and blockages in passive reactive barrier technologies does not occur;
- No surface coating or deactivation particularly in passive reactive barrier technologies occurs;
- Limited carbon footprint as Bauxsol™ is derived from an industrial by-product, therefore the Alumina industry has already generated the footprint;
- Creates a thin sediment not a gelatinatenous sludge, which requires no further treatment or disposal;
- Low reaction pH’s (<9.0) poses a minimal health and safety risk;
- Binds up to 99.99% of trace-metals that meet TCLP Standards.
